The Ion Exchange Resin process (IER) are usually considered as similar to electrolytes. The IER were developed in the middle 1940’s and it is based on the co-polymerization of styrene cross-linked with divinylbenzene.
Basic Concepts of Ion Exchange Resin Process for Sugar Refinery
Basic Ion Exchange Process
The IER system involves one ion for another, hold it temporarily, and then release it to a regenerate solution in regeneration process.
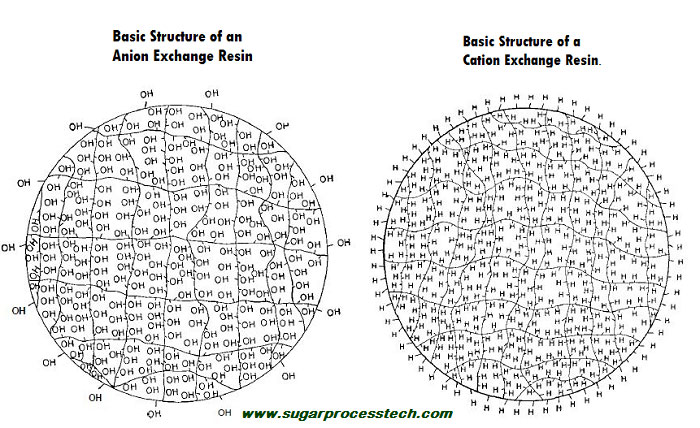
The basic polymeric structure is of IER is called the matrix. Functional groups – acidic, basic or salt – are attached to the matrix. The matrix along with the functional group is called a functional polymer or ion exchanger or simply ion exchange resin.
Ion Exchange Resin = Polymer Matrix + Functional Groups
The basic polymer structure is called matrix on which active functional groups are attached. Most are manufactured used matrix as styrenic or acrylic polymers and are chemically activated with various functional groups.
Cation-exchange resins are activated by negative functional groups such as sulfonates (- SO3H) or carboxyl (-COOH), and anion-exchange resins are activated by positive functional groups such as quaternary ammonium. The resin structure have been modified in many ways like macroreticular, or macroporous or Gel as per the specific applications and to meet the requirements.
For a strong cationic -exchange resin
NaCl + H-R ↔ NaCl-R + HCl
For a strong anionic-exchange resin,
NaCl + OH-R ↔ Cl-R + NaOH
Styrenic resins are able to remove colorants through ionic bonds with resin cationic active groups as well as hydrophobic by reactions with the resin matrix.
Ion Exchange Resins Types
Strong Acid Cation (SAC) :
The Strong Acid Cation resin has a styrene-divinyl benzene matrix with sulphonic acid functional group. The standard SAC resin has a divinyl benzene (DVB) content of 8%. Resins with divinyl benzene content below and above the standard are available for different applications. The resin matrix may have a gel or macroporous structure. The SAC resin exchanges all cations in water and is used in the softening, dealkalization and demineralization of water.
Weak Acid Cation (WAC):
The Weak Acid Cation resin consists of polyacrylic acid – divinyl benzene matrix with carboxylic acid functionality and a gel structure. The WAC resin exchanges all cations associated with alkalinity in water and is noted for its high capacity for the divalent cations. The resin is characterized by a high efficiency of regenerant utilization. The resin is used therefore primarily for the dealkalization of water. It also finds use as a first stage in a demineralization train.
Strong Base Anion (SBA):
The Strong Base Anion (SBA) resin has a styrene-divinylbenzene matrix with a gel or macroporous structure. The SBA resin with an isoporous structure does not use DVB as a cross linking agent. Instead EDMA (ethylene dimethyl methacrylate) is the cross linking material used and which provides uniform porosity. The SBA resin has quaternary ammonium functionality.
Weak Base Anion (WBA):
The Weak Base Anion resin has a styrene-divinylbenzene matrix with a macroporous structure and tertiary ammonium functionality. The WBA resin is noted for its high capacity for free mineral acidity and high efficiency of regeneration. The resin is used downstream of a SAC resin and ahead of a SBA resin in a demineralization scheme to bring about economy of operation.
Particle Size of Ion Exchange Resins
Particle size distribution is an important physical parameter of a resin affecting such factors as backwash expansion, pressure drop characteristics and kinetics. Coarse particles yield lower pressure drop and a poorer rate of exchange. Fine particles result in higher pressure drop but a better rate of exchange. Resins are manufactured in a size range from 0.3 mm to 1.2 mm. Particles smaller than 0.3 mm are termed as ‘fines’.
Ion Exchange Resin Process Mechanism of colour removal in melt liquor in sugar refinery
This Ion-exchange technology was introduced into sugar process in the 1970’s and it is one of the best technology for melt decolorization in sugar refinery process.
Ion exchange reaction in between negatively charged colorants and hydrophobic interaction between the non polar colorants with adsorption inside the pores of the resin bed of colorants.
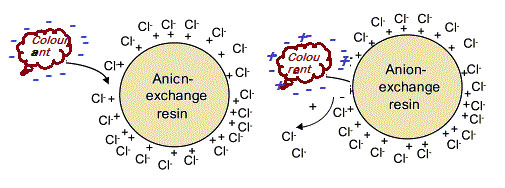 When in contact with a sugar melt solution to the ion-exchange resin reacts according to a simple acid-base chemical reaction.
When in contact with a sugar melt solution to the ion-exchange resin reacts according to a simple acid-base chemical reaction.
In the sugar industry the resins used are strong base anion (SBA) exchange resins in the chloride form with a resin matrix of two types either styrenic or acrylic. Because most of the sugar colorants are anionic in nature, being negatively charged at high pH. So strong base anionic resins are efficient decolorizers for sugar melt.
Strong base anion (SBA) exchange resins derive their functionality from quaternary ammonium functional groups. It having two types of quaternary ammonium groups named as a Type I & Type II.
Type I sites have three methyl groups of trimethylamine, which yields chloride ions (Cl–), and Type II resin is one of the methyl groups in type I is replaced with an ethanol group as dimethylethanolamine, which yields hydroxide ions (OH–). Type II resins has a less stability than the Type I resin and Type -I is able to remove more of the weakly ionized acids.
| S.NO | Description | Styrenic Macroporous | Acrylic Macroporous |
| 1 | Primary Decolorization Mechanism | Adsorption | Ion Exchange |
| 2 | Matrix | Aromatic – Polystyrene, macroporous reticulated with divinybenzene | Aliphatic – Polyacrylic, macroporous reticulated with divinybenzene |
| 3 | Functional Groups | strong base type with three methyl groups R -N+(CH3)3 | Quaternary ammonium,strong base |
| 4 | Typical Decolorization | 65-75% | 50-60% |
| 5 | Regenerant | 10% NaCl/0.5% NaOH | 10% NaCl |
| 6 | Color Loading BV x IU (68 Bx) | 25000 | 35000 |
| 7 | Remarks | Polystyrenic is cheaper but less life, good for low colour loading. | Poly acrylic is expensive gives higher life, good for high colour loading but high hydraulic resistance. |
Acrylic resins are less hydrophobic interaction with nonpolar parts of colorants and it is more resistant than styrenic resins to high color feed levels. At the same time the styrenic resins have a higher decolorization rate and better capacity with lower color feed levels.
Microscopic view of a macroporous strong base anion resin
Related Articles
Overview of Refined Sugar Process
Different Methods of Decolourization of Raw melt
Syrup Clarification System and Melt Clarification System
Design Criteria of Melt Clarification System
Carbonation Process in Sugar Refinery
Ion Exchange Process for melt decolonization of sugar refinery
Color, Solids and Purity of Crystallization in Sugar Refinery Process
Material Balance Calculation for three massecuite Boiling
Material balance of three and half massecuite boiling calculation
Thumb Rules for Sugar factory Equipment Design Sizing