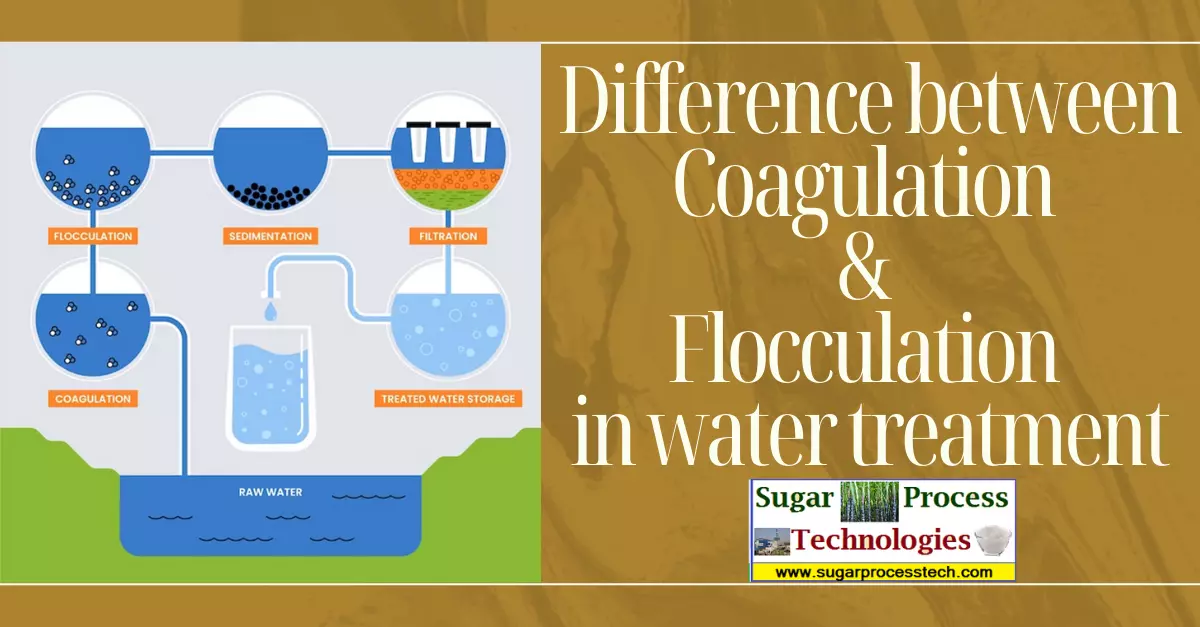
Coagulation and flocculation are two essential processes in water and wastewater treatment that work together to remove impurities, suspended particles, and contaminants from the water. While they are closely related, they serve distinct purposes in the treatment process. Let’s break down the differences between coagulation and flocculation.
Before we break down the differences between these two terms, we know about the process of Coagulation and flocculation
Need for Coagulation and Flocculation processes in water treatment:
The need for coagulation and flocculation in the water treatment process because some impurities in water are extremely tiny. They’re so small that they can’t be removed just by letting them settle or by using filters. To remove this, we add special chemicals. These chemicals make these tiny particles unstable, causing them to stick together. This process, where we add these chemicals, is called coagulation. Then comes flocculation, which is when we gather these unstable, clumped-together particles to form bigger groups, called flocs. This makes it much easier to remove them from the water. So, coagulation and flocculation are key steps in the water treatment process.
Coagulation:
Coagulation is the first step in making a messy room tidy. Imagine your room is filled with scattered toys, and you want to group them. Coagulation is the process of bringing tiny impurities in the water, like dust particles, bacteria, and other small bits, together so they can be easily managed.
Coagulation is the initial step in the treatment process, and its primary goal is to neutralize the electric charges of small, suspended particles and colloids in the water or wastewater.
How Coagulation works:
- Chemical Addition: In coagulation, specific chemicals called coagulants are added to the water or wastewater. Common coagulants include ferric chloride, aluminum sulfate (alum), and ferric sulfate.
- Charge Neutralization: These coagulants contain ions that neutralize the negative charges on suspended particles. As a result, the particles lose their repulsive forces, allowing them to come together and form smaller aggregates.
- Formation of Microflocs: The neutralized particles initially form tiny aggregates known as microflocs. While these microflocs are larger than individual particles, they are still too small to settle out of the water effectively.
Flocculation:
Now, think of flocculation as the second step in making your room neat. After you’ve grouped your toys into small clumps, you want to make these clumps bigger and easier to handle. Flocculation does just that.
Flocculation follows coagulation in the treatment process and focuses on bringing the microflocs created during coagulation together to form more substantial particles, known as flocs.
How Flocculation works:
- Gentle Agitation: Instead of adding chemicals, flocculation involves gently stirring the water or wastewater in a flocculation basin. This gentle agitation promotes collisions between microflocs. i.e. This stirring action helps the small clumps created during coagulation come into contact with each other.
- Floc Formation: As microflocs collide and come into contact with each other, they bond together to create larger and denser flocs. The physical mixing action in the basin facilitates this process. i.e. these clumps bump into each other, they stick together, just like when you push two snowballs together, they become a bigger snowball. Over time, as these clumps keep bumping into each other, they grow larger and larger.
- Increased Settling Efficiency: The resulting flocs are much larger and heavier than individual particles, making them more likely to settle out of the water by gravity during the next treatment steps, such as sedimentation or filtration.
Key Differences Between Coagulation and Flocculation Proces :
- Purpose:
- Coagulation: The primary purpose of coagulation is to neutralize the electric charges of small suspended particles and colloids in the water. This neutralization initiates particle aggregation.
- Flocculation: In contrast, flocculation focuses on bringing together the already coagulated particles, known as microflocs, to form larger, denser particles called flocs. Its aim is to enhance the size and settleability of these particles.
- Chemical vs. Mechanical:
- Coagulation: Coagulation involves the addition of specific chemicals called coagulants. These coagulants contain ions that neutralize the negative charges on suspended particles.
- Flocculation: Flocculation, on the other hand, depends on mechanical means, such as gentle stirring or mixing in a flocculation basin. It doesn’t involve the addition of chemicals but rather utilizes physical agitation.
- Particle Size:
- Coagulation: Coagulation forms microflocs by neutralizing charges on small particles. These microflocs are larger than individual particles but still too small to settle effectively.
- Flocculation: The main goal of flocculation is to bring these microflocs together to create significantly larger and denser flocs. These flocs are visible and have a much higher settling efficiency.
- Agitation Intensity:
- Coagulation: Coagulation doesn’t require intense agitation. Its primary function is to initiate the process of particle aggregation by reducing electrostatic repulsion.
- Flocculation: Flocculation involves gentle, continuous mixing within a basin. This mechanical agitation encourages collisions between microflocs, promoting their bonding and growth.
- Efficiency:
- Coagulation: Coagulation is the initial step that sets the stage for subsequent processes but doesn’t lead to significant settling of particles on its own.
- Flocculation: Flocculation significantly increases the settling efficiency of particles. It prepares them for subsequent treatment steps, such as sedimentation or filtration, by forming larger, more easily separable flocs.
In summary, coagulation and flocculation are initial processes in water and wastewater treatment. Coagulation initiates the formation of microflocs, while flocculation facilitates the growth of these microflocs into larger, settleable flocs. Together, they improve the efficiency of downstream treatment processes and ensure the removal of impurities from the water.
Related Articles:
Classification of Impurities Present in Water Related to Industrial Application
Chemical Oxygen Demand (COD): Meaning, Applications, and Control Strategies
Basic concepts of Raw Water treatment system used in industrial applications
ETP | Sugar industry effluent treatment plant process philosophies
Humidity, Relative Humidity, Absolute Humidity, Specific Humidity & Dew Point
Activated Sludge Process for Wastewater Treatment | Anaerobic Digestion
Anaerobic Treatment Process for Industrial Waste Water | Anaerobic digester
CSTR | Continuous Stirred Tank Reactor Design Criteria | Anaerobic Digester