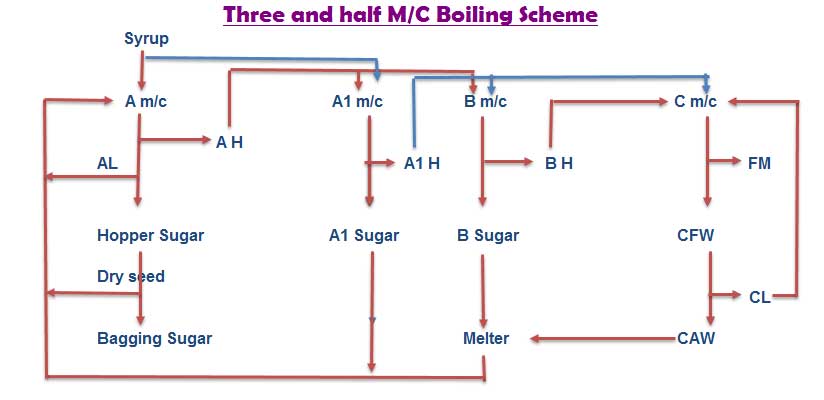
Sugar Industry Three and half massecuite boiling scheme material balance
Planitation white sugar industry process three massecuite boiling scheme is not giving lower purity of C massecuite and final molasses. In three massecuite boiling scheme also difficult to getting maximum ” M ” size sugar. In this situation it is better to go four massecuite boiling scheme. But due to installed equipments does not permit to follow four massecuite boiling scheme, then a short alternative of half massecuite either between A and B masscuite or B and C massecuite is followed. Then the boiling scheme is called as “Three and half massecuite” & the intermediate massecuite is called as A1 or B1 or C1 massecuite depending on it’s graining material used.
Here given one example for three and half massecuite boiling scheme.
Sugar Factory Material Balance Calculation for 3 m/c Boiling
Take one example for the three and half massecuite boiling scheme calculation:
Collect the following data from lab analysis
Calculate the syrup solids from the below formulas.
1. Solids in syrup%cane = Solids in Clear juice % cane.
2. Solids in Clear juice % cane = Brix in clear juice x Clear juice % cane.
3. Brix in Clear juice = Pol in clear juice / Purity in clear juice.
4. Purity in clear juice = Calculate by the laboratory method.
5. Pol in clear juice = Pol in Mixed juice % cane – Pol in filter cake % cane.
| Material Name | Brix | Purity |
| SYRUP | 56.00 | 85.00 |
| A m/c | 92.00 | 90.00 |
| A1 m/c | 93.00 | 80.00 |
| B m/c | 95.00 | 72.00 |
| C m/c | 99.00 | 56.00 |
| AH | 80.00 | 76.00 |
| AL | 75.00 | 91.00 |
| A1H | 77.00 | 65.00 |
| BH | 85.00 | 51.00 |
| CL | 78.00 | 65.00 |
| White Sugar | 100.00 | |
| A1 sugar | 99 | 98.00 |
| B sugar | 96.00 | 94.00 |
| CFW | 93.00 | 80.00 |
| CAW | 95.00 | 93.00 |
| FM | 88.00 | 30.00 |
| AFW | 99.40 | |
Step 1 : Find the Solids in Sugar and Final molasses.
| 100.00 | Sugar | 85 – 32 = | 53.00 | ||
| Syrup | 85.00 | ||||
| 32.00 | FM | 100 – 85 = | 15.00 | ||
| 68.00 |
If syrup solids divided into 68 parts , 53 solids will be in sugar and 15 solids will be in final molasses.
Solids in Unknown 1 = ( Parts of Unknown 1 / Parts of known fraction ) x Solids in known fraction.
Final sugar solids (Solids in sugar) = (53 x 15)/68 = 11.69.
Solids in FM= Solids in syrup – Solids in sugar =15 – 11.69= 3.31.
Step 2 : Find the Solids in C massecuite and CFW( C Fore Worker).
| 80.00 | CFW | 24.00 | ||
| C m/c | 56.00 | |||
| 32.00 | FM | 24.00 | ||
| 48.00 |
Solids in C m/c = (48 /24) x 3.31 = 6.62
Solids in CFW = Solids in C m/c – Solids in Final Molasses = 6.62 – 3.31 = 3.31.
Step 3 : Find the Solids in C Light and CAW( C After Worker).
| 93.00 | CAW | 15.00 | ||
| CFW | 80.00 | |||
| 65.00 | CL | 13.00 | ||
| 28.00 |
Solids in CAW = ( 15/ 28) x 3.31 = 1.77
Solids in C Light = Solids in CFW – Solids in CAW = 3.31 – 1.77 = 1.54.
Step 4 : Find the Solids from B Heavy for C m/c boiling and solids from A1 heavy for C m/c Boiling.
C M/C boiling with A1H , BH and CL.
Here we know only CL. So Take (A1H + BH ) imaginary mixture for C m/c boiling.
Solids in A1H + BH = Solids in C m/c – Solids in CL = 6.62 – 1.54 = 5.08
[ C m/c solids x C m/c pty ] = [ (A1H+BH) solids x (A1H+BH) pty ] + [ CL solids x CL pty ]
Imaginary mixer (A1H+BH) Purity ={ [ C m/c solids x C m/c pty ] – [ CL solids x CL pty ] } / (A1H+BH) solids.
Imaginary mixer (AH+BH) Purity = { [ 56 x 6.62 ] – [ 65 x 1.54] } / 5.08 = 53.28.
| 65.00 | A1H | 2.28 | ||
| A1H+BH | 53.28 | |||
| 51.00 | BH | 11.72 | ||
| 14.00 |
Solids in B Heavy for C boiling = ( 11.72 / 14) x 5.08 = 4.25
Solids from A1H for C boiling = 5.08 – 4.25 = 0.83.
Step 5 : Find the Solids in B m/c and B sugar.
| 94.00 | B sugar | 21.00 | ||
| B m/c | 72.00 | |||
| 51.00 | BH | 22.00 | ||
| 43.00 |
Solids in B m/c = ( 43/ 22) x 4.25 = 8.31
Solids in B Sugar = Solids in B m/c – Solids in BH = 8.31 – 4.25 = 4.06.
Step 6 : Find the Solids in A1H and AH for B m/c .
| 65.00 | A1H | 4.00 | ||
| B m/c | 72.00 | |||
| 76.00 | AH | 7.00 | ||
| 11.00 |
Solids in A Heavy for B m/c boiling = (7/11) x 8.31 = 5.29
Solids in A1H for B m/c boiling = 8.31 – 5.29 = 3.02.
Total solids in A1 H = Solids in A1H for B m/c + solids in A1H for C m/c = 3.02 + 0.83 =3.85.
Step 7 : Find the Solids in A1 m/c and A1 sugar.
| 98.00 | A1 sugar | 15.00 | ||
| A1 m/c | 80.00 | |||
| 65.00 | A1H | 18.00 | ||
| 33.00 |
Solids in A1 m/c boiling = (33/18) x 3.85 = 7.06
Solids in A1H for B m/c boiling = 7.06- 3.85 = 3.21.
Step 8 : Find the Solids in AH and Syrup for A1 m/c boiling.
| 85.00 | Syrup | 4.00 | ||
| A1 m/c | 80.00 | |||
| 76.00 | AH | 5.00 | ||
| 9.00 |
Solids in A Heavy for A1 m/c boiling = (5/9) x 7.06 = 3.92
Solids in Syrup for A1 m/c boiling = 7.06 – 3.92 = 3.14.
Total solids in AH = Solids in AH for A1 m/c + solids in AH for B m/c = 3.92 + 5.29 = 9.21.
Step 9 : Find the solids in A m/c and AFW
| 99.40 | AFW | 14.00 | ||
| A m/c | 90.00 | |||
| 76.00 | AH | 9.40 | ||
| 23.40 |
Solids in A m/c = (23.4/9.4) x 9.21 = 22.93.
Solids in AFW = Solids in A m/c – Solids in AH = 22.93 – 9.21 = 13.72.
Step 10 : Find the solids in Hopper Sugar and A Light.
| 100.00 | Hopper | 8.40 | ||
| AFW | 99.40 | |||
| 91.00 | AL | 0.60 | ||
| 9.00 |
Solids in Hopper sugar = (8.4/9) x 13.72 = 12.80.
Solids in AL = Solids in AFW – Solids in Hopper Sugar = 13.72- 12.80 = 0.92
Step 11 : Find the solids in Dry Seed.
Solids in dry seed = Solids in hopper sugar – Solids in bagging sugar =13.72 – 11.69 = 2.03.
Step 12 : A m/c Solid balance. (This step help to cross check the calculation)
| Solids | Purity | Solids x Purity | |
| Syrup | 11.86 | 85.00 | 1008.3 |
| A1 sugar | 3.21 | 98.00 | 314.5 |
| Dry Seed | 1.12 | 100 | 111.6 |
| AL | 0.91 | 91.00 | 83.2 |
| B Sugar | 4.06 | 94.00 | 381.7 |
| CAW | 1.77 | 93.00 | 164.9 |
| Total | 22.94 | 2064.23 |
Expected A m/c Purity = 2064.23/ 22.94 = 89.98. ( Given A m/c purity is 90. So our calculation having no mistakes)
Step 13 : Calculate all material % cane
Material % Cane = Solids in material x 100 / Brix % material
| Material | Solid% | Brix% | %Cane |
| SYRUP | 15.00 | 56.00 | 26.79 |
| A m/c | 22.94 | 92.00 | 24.93 |
| A1 m/c | 7.06 | 93.00 | 7.59 |
| B m/c | 8.32 | 95.00 | 8.75 |
| C m/c | 6.62 | 99.00 | 6.68 |
| AH | 9.21 | 80.00 | 11.52 |
| A1H | 3.85 | 77.00 | 5.00 |
| AL | 0.91 | 75.00 | 1.22 |
| BH | 4.25 | 85.00 | 5.00 |
| CL | 1.54 | 78.00 | 1.97 |
| A1 Sugar | 3.21 | 99.00 | 3.24 |
| B sugar | 4.06 | 96.00 | 4.23 |
| CFW | 3.31 | 93.00 | 3.56 |
| CAW | 1.77 | 95.00 | 1.87 |
| FM | 3.31 | 88.00 | 3.76 |
Some related articles
Solid Balance in Sugar industry process | Material Balance Calculation , Sugar Factory Material Balance Calculation for 3 m/c Boiling
Sugar Industry Related Important Websites | sugar Technology
Calculator to Find liquid volume for vertically mounted cylindrical volume and also find the volume in cylindrical tank partitioned portion.
Formula for evaluating Horizontal Cylinder volume at different levels (vacuum crystallizer volume at different sight).
Hi friends Thanks for reading the Three and half massecuite boiling material balance calculation . I Hope you liked it. Give feed back, comments and please share it.

34 thoughts on “Three and half massecuite boiling material balance calculation |Sugar Tech”
Revansidha.K.patane
(November 18, 2017 - 8:46 am)Tks vv good imf tks
siva alluri
(November 20, 2017 - 4:14 am)Thank you sir
please share your sugar tech friends
MANDHATA PATEL
(November 20, 2017 - 4:35 am)thanks sir,. nice work
ALOK KUMAR DWIVEDI
(November 21, 2017 - 7:35 am)You have written so beautifully but it will not follow in up region as I calculated
siva alluri
(November 21, 2017 - 8:20 am)Ok kumar sir,
suggest the your boiling scheme, what your region follows
Ranjit kawade
(December 8, 2017 - 1:19 am)So Nice Information in this Website.
Thank you so much.
I Have also Completed Bsc (Sugar Technology)
Rajarambapu institute of technology Islampur
siva alluri
(December 8, 2017 - 1:56 pm)This website invite to all sugar technologists to share knowledge
sandesh patil
(January 23, 2018 - 5:47 pm)good information in this web side
i also pass out in rajarambapu institute of suger technology islampur
siva alluri
(January 25, 2018 - 8:09 am)Thank you
Vijaykumar Dure
(March 25, 2018 - 8:46 am)R / sir this site is very very Good learning about sugar plant for overall calculation means fully basic terms Thanks so much .
siva alluri
(March 25, 2018 - 5:19 pm)Thank you Mr. Vijaykumar
Javier Ibáñez
(April 12, 2018 - 4:34 pm)Este site es muy bueno…felicitaciones!
Javier Ibáñez
(April 12, 2018 - 4:37 pm)I would like to know if you have material to calculate and make management in difussers.
siva alluri
(April 16, 2018 - 1:24 pm)We will published an article on diffusers soon
Yibeltal tesfa
(April 18, 2018 - 8:19 am)Wow I am very impressive on your notes, but I have a question for u I am not understand on the difference b/n three and half boiling and four boiling scheme based on there operational system
siva alluri
(April 19, 2018 - 3:38 pm)Thank you Mr.Yibeltal tesfa
The main difference between 3 1/2 & 4 m/c boiling scheme is
The entire molasses diverted to next massecuite without deviation upto end ( it may take little bit high purity material for purity balance )
I.e Syrup to A m/c → A Heavy molasses to B m/c → B molasses to C m/c → C molasses to D m/c Then it is called 4 massecuite boiling
For purity balance purpose (FM purity) or crystal size purpose or quality purpose some times introduces one massecuite boiling in-between two massecuites with partial quantity molasses
I.e suppose B2 m/c introduced in-between A and B massecuites then
AH diverted to B2 m/c as per requirement purity and remaining AH diverted to B m/c. So the complete AH molasses not diverted to B2 m/c
So it is called 3 1/2 massecuite.
Vilas patil
(August 15, 2021 - 12:02 pm)Thanks sir very very nice information
siva alluri
(September 2, 2021 - 8:32 am)welcome Mr.patil
UDAI VEER
(August 12, 2018 - 12:57 am)Thks to all 9480137343
Ram veer
(October 23, 2019 - 3:48 am)3 and half m/c boiling me c1 m/c banane ke liye material balance method bataye sir
manoj kumar gupta
(October 31, 2019 - 5:18 am)I want sugar refinery solid and water balance if you can provide.
siva alluri
(October 31, 2019 - 6:27 am)Please go through the below link for Solids, Purity & Color Balance of Refined Sugar Massecuite Boiling
https://www.sugarprocesstech.com/refined-sugar-massecuite-boiling/
S K Mishra
(November 18, 2019 - 6:31 am)R/sir,
Can u provide colour balance of plantation white sugar process from cane to final sugar with reduction percentage along with sugar colour methods. Regards
siva alluri
(November 18, 2019 - 12:05 pm)Ok we will provide soon for plantation white sugar
Already provided for refined sugar
https://www.sugarprocesstech.com/refined-sugar-massecuite-boiling/
Surendra Singh
(February 12, 2020 - 8:28 am)Thanks dear for important knowledge,
Dear sir,
I want solid balance in that condition While we divert 50% quantity of BH molasses to distillery, then
Recovery loss = ?
Decreased C-m/C quantity =?
Losses =?
Atul Krishna Jadhav
(September 10, 2020 - 6:15 am)Please publish S/V Calculation for A/B/C Conti.pan
Also Evaporation Rate calculation for Pans
siva alluri
(September 11, 2020 - 4:37 am)Go through the below links
https://www.sugarprocesstech.com/pan-section-capacity/
https://www.sugarprocesstech.com/vacuum-pan-sugar/
https://www.sugarprocesstech.com/hydrostatic-pressure/
https://www.sugarprocesstech.com/graining-volume/
Aniket Bhave
(October 1, 2020 - 8:47 am)Dear Sir,
Please provide colour balance from mixed juice to final sugar with reduction percentage along for double sulphitation process for production of plantation white sugar.
siva alluri
(October 2, 2020 - 1:49 pm)Ok we will provide soon
M.Homsie
(December 5, 2020 - 7:14 am)Can I get a good method in which I can regulate the use of steam in crystalization processes?
belekar pramod
(September 20, 2023 - 5:19 am)Dear sir,
pl.provide two massecuite boiling solid balance( all B-Heavy molasses diversion to distillery ) with colour balance.
siva alluri
(September 20, 2023 - 1:48 pm)Ok we will provide
Shakeel Sarwar
(November 29, 2023 - 6:49 am)Very fine knowledge
siva alluri
(December 1, 2023 - 5:27 am)welcome