This Article summarizes the concepts of final molasses generation in process, Properties Molasses like viscosity, specific heat.Also discussed to overcome the issue of deterioration during storage. Molasses cooler design concepts and its water requirement calculation.
Final Molasses Storage | Shell and Tube Molasses Cooler Design Concepts
Final Molasses is one of the important & valuable byproduct of the sugar factory. Its quantity is generally upto 4 – 5 % of the cane crushing capacity. It is a common practice that in all of the sugar factories the final molasses produced during the crushing season is stored in the storage tank. This storage tank is called as molasses storage tank.
Parameters of final molasses
pH – 4.5 to 5.5
Brix (solids) – 88 to 92%
Sucrose(Pol) – 25 – 32%
Reducing sugar – 12 -18%
Nitrogen % – 0.15 – 0.25
Gums % – 0.5 – 3.5
CaO % – 1.0 – 1.5
SO4 % – 1.2 – 3.5
P2O5 % – 0.25 – 0.3
Volatile Acidity – Below 3000 ppm
Total Reducing Sugars (TRS) – 40 to 55%
Unfermentable sugars – 4 to 6%
Molasses deterioration is a common phenomenon during storage in the tanks. Molasses has a tendency to undergo rapid destruction & ultimately leading to know about spontaneous combustion. The phenomenon of spontaneous combustion occurs purely by virtue of certain chemical reaction. Basically it is an “exothermic reaction”.
This combustion is due to the reaction of unstable organic substances (Originally produced by the action of lime upon the reducing sugar of cane during clarification ) with further quantities of reducing sugar in molasses which results in the formation of dark coloured colloidal impurities of high carbon content.
The deterioration of the molasses through the Maillard reactions leading to catastrophic consequences.
Maillard reactions can be summarized as:
Reducing sugars + Amino acids → Melanoidins
The Maillard reactions are initiated/accelerated with higher temperatures during storage with the release of gases (mainly carbon dioxide) and foaming in the molasses tanks.
Factors responsible for spontaneous combustion of molasses:
In case of certain factories when the low grade massecuite are boiled at a little high temperature. The certain ingredients of molasses are over heated & thus causing higher carbon content.
After centrifuge the the final molasses sent to storage tanks with high temperature means without cooling it.
The open tank in which the molasses is stored are exposed to the direct heat from the sun. With the direct heat of sun entering in the molasses, the combustion is helped by the higher temperature of the heat of the sun particularly in the month of summer.
Precaution to Avoid Spontaneous Combustion of Molasses:
The quality of the molasses in storage tanks should be regularly monitored with a check on the total reducing sugars. The deterioration of molasses due to chemical reactions during storage can be avoided by proper cooling of molasses to ambient temperatures prior to storage. The molasses temperature (inlet) should below 45 oC
There must be throughout cold water recirculation arrangement around the wall of the tank in day of summer season.
Appropriate venting in the molasses stored tank.
Physical Properties Molasses
Viscosity of Molasses:
Viscosity of a Newtonian liquid is measured as the ratio of shear stress to shear rate.
Where is the shear stress (force/unit area, Pa) &
the shear rate in S -1
If a viscometer is used to measure the consistency of a non-Newtonian fluid, the relationship between shear stress and shear rate is the apparent viscosity.
The viscosity of the molasses, which depends on its temperature and purity.
The viscosity of molasses increases rapidly as its temperature decreases. It is approximately three times as high at 40°C as it is at 50°C.
Viscosity increases’ equally rapidly with the brix.
Viscosity also increases with the proportion of air entrapped in the form of fine bubbles in the molasses. For example with 10% and 20% by volume of entrapped air, the viscosity may be respectively 15% and 50% higher than it would be in the absence of included air.
Dynamic viscosity of pure sucrose solutions in mPa • s (CP) (Bubniketal 1995)
| Sucrose content in % | Temperature in oC | ||||||||
| 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | |
| 0 | 1.76 | 1.3 | 1 | 0.8 | 0.65 | 0.55 | 0.47 | 0.42 | 0.37 |
| 10 | 2.22 | 1.65 | 1.29 | 1.04 | 0.85 | 0.7 | 0.6 | 0.51 | 0.45 |
| 20 | 3.78 | 2.64 | 1.95 | 1.49 | 1.18 | 0.97 | 0.81 | 0.68 | 0.59 |
| 30 | 6.69 | 4.49 | 3.19 | 2.37 | 1.83 | 1.47 | 1.2 | 1 | 0.85 |
| 40 | 14.6 | 9.17 | 6.17 | 4.38 | 3.24 | 2.49 | 1.97 | 1.6 | 1.32 |
| 50 | 44.7 | 25.2 | 15.4 | 10.1 | 6.99 | 5.07 | 3.81 | 2.94 | 2.34 |
| 60 | 236 | 111 | 58.5 | 33.8 | 21 | 14 | 9.66 | 6.98 | 5.2 |
| 62 | 365 | 163 | 82.4 | 46 | 27.8 | 17.9 | 12.2 | 8.63 | 6.35 |
| 64 | 592 | 249 | 120 | 64.5 | 37.7 | 23.7 | 15.7 | 10.9 | 7.87 |
| 66 | 1013 | 399 | 182 | 93.5 | 52.6 | 32.1 | 20.6 | 14.1 | 9.93 |
| 68 | 1846 | 672 | 289 | 141 | 76 | 44.7 | 27.9 | 18.4 | 12.8 |
| 70 | 1206 | 482 | 222 | 114 | 64.4 | 39 | 25 | 16.8 | |
| 72 | 368 | 179 | 96.5 | 56.1 | 34.9 | 22.8 | |||
| 74 | 296 | 152 | 84.1 | 50.3 | 32 | ||||
| 76 | 247 | 131 | 76.7 | 45.9 | |||||
| 78 | 221 | 122 | 70.5 | ||||||
Note: 1 centi Poice = 1 mPa.s
An impure solution of sucrose has a viscosity much higher than that of a pure solution of the same brix, and very variable according to the nature of the impurities. In the absence of better data, an approximate value for the viscosity of a solution of purity “P” may be deduced from that of a pure solution of the same brix by taking
Specific heat of Molasses
As per the Cane Sugar Engineering by Peter Rein – The specific heat of molasses is typically about half that of water. And proposed the following equation
Where Cp = Specific heat of the molasses in KJ/kg/oC
B = Brix % molasses ( % of Dry solids)
Density of Molasses :
This varies according to the dissolved solids content and the degree of aeration. It usually, a value between 1400 and 1450 kg/m3 when not aerated and @ 85 to 90 brix.
Types of Molasses Cooling System
Mainly using two types of designs are used for molasses cooling and they are
1 .Horizontally located shell and tube heat exchanger
2. Plate type heat exchanger.
Plate Type cooler for molasses:
Heat transfer rates are high in plate type design when compare to shell and tube design. So plate type heater coolers are compact and relatively less expensive to install.
Moreover viscosity has a substantial effect on performance of plate type design. Due to high viscosity of molasses and pressure drops, plate type cooler is not a successful design for this application.
However some designers modified in plate patters of cooler to overcome the drawbacks of this system.
Shell and tube cooler for Molasses:
Shell and tube is one of the successful design for molasses cooling application.
Tubular cooler with water inside the tubes – In this design involves the flow of molasses over banks of small diameter tubes through which cooling water flows.
i.e Molasses passes through shell side of the heater and water flows through the tubes.
Generally, water side scale formation is high when compare to molasses side according to their properties so it will be the main advantage for proper cleaning.
Proper baffles arrangement in shell side is also important criteria for this type system.
But in this system heat transfer rates are low when compare to other type systems. Another drawback is pressure drops is higher.
Tubular cooler with molasses inside the tubes – In this design involves the flow of water over banks of small diameter tubes through which molasses flows.
This is a common design particularly when old juice heaters are available. Duplex type design will be the more effective solution for this type of design
Velocity of molasses in the tubes is expected around 0.20 m/sec. to 0.25 m/sec.
Heat transfer coefficients (HTC) for molasses coolers:
HTC plays an important role in the heater and coolers heating surface calculation. Heat transfer coefficient depends on the following parameters
Molasses film resistance (hot media),
Cold water (cold media),
Tube resistance – According to material of construction (MOC) of the tube
Resistance of scales ( fouling factor)
Plate type heaters – 350 to 450 Kcal/ hr/ m2/oC
Tubular cooler with water inside the tubes – 40 to 90 Kcal/ hr/ m2/oC
Tubular cooler with molasses inside the tubes – 80 to 130 Kcal/ hr/ m2/oC
Molasses Cooler Heating Surface Calculation:
Basic Cooling surface formula
Qm x Cm x ∆T = K x S x ∆Tm – – – ( 1)
Where
S = Molasses cooler heating surface in m2
Qm = Flow rate of the Molasses in T/hr
Cm = Specific heat of molasses in Kcal/kg/0C
Ti =Molasses inlet temperature in 0C
To = Molasses outlet temperature in 0C
ti = Water inlet temperature in 0C
to= Water Molasses outlet temperature in 0C
K = Heat Transfer Coefficient in Kcal/ hr/ m2/oC
∆T = Ti – To
∆Tm = ∆Ti -∆ Te / ( ln(∆Ti/∆Te))
∆Ti = Ti – to
∆Te = To – ti
Take One example for calculation
| S.No | Particulars | Values | UOM | Remarks |
| 1 | Qm = Molasses flow rate | 15.00 | T/hr | |
| 2 | K = heat transfer coefficient | 80 | kcal/m2/hr/0C | |
| 3 | Molasses Brix | 88 | % | |
| 4 | Cm | 0.38 | Kcal/kg/oC | 1 – 0.007* Brix |
| 5 | Ti | 60 | oC | |
| 6 | To | 45 | oC | |
| 7 | ti | 35 | oC | |
| 8 | to | 40 | oC | |
| 9 | ∆T | 15 | oC | ∆T = Ti – To |
| 10 | ∆Ti | 20 | oC | ∆Ti = Ti – to |
| 11 | ∆Te | 10 | oC | ∆Te = To – ti |
| 12 | Ln(∆Ti/∆Te) | 0.69315 | ||
| 13 | ∆Tm | 14.43 | ∆Tm = ∆Ti -∆ Te / ( ln(∆Ti/∆Te)) |
The usual practice in the design of shell and tube exchangers is to estimate the “true temperature difference” from the logarithmic mean temperature by applying a correction factor to allow for the departure from true counter-current flow
The usual practice in the design of shell and tube exchangers applying a correction factor for true counter-current flow
∆Tm (true) = Ft x ∆Tm
∆Tm (true) = True temperature difference, the mean temperature difference for use in the above Cooling surface formula.
Ft = the temperature correction factor.
The correction factor is normally correlated as a function of two dimensionless temperature ratios
R is equal to the ratio of “shell-side fluid flow-rate times the fluid mean specific heat ” to “tube-side fluid flow-rate times the tube-side fluid specific heat”.
S = the temperature efficiency of the exchanger.
The correction factor is given by Kern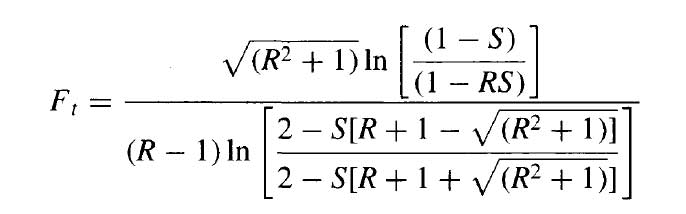
The equation for a one shell and two tube pass exchanger can be used for any exchanger with an even number of tube passes.
Now substitute the above values in this equation then
Ft = 0.85
∆Tm (true) = 14.43 x 0.85 = 12.26
Now substitute all these values in equation ( 1) i.e Cooling surface formula – Qm x Cm x ∆T = K x S x ∆Tm
Then
Heating surface of the molasses cooler (S )= 88 m2
Cold water requirement
W = Qm x Cm x (Ti-To)/ Cw (to- ti) – (2)
W = Flow rate of water in M3/hr
Cw = Specific heat of water = 1 Kcal/kg/0C
Now substitute values in the equation ( 2) then
W = 17.5 M3/hr
Now according to above values we can design the shell and tube cooler easily by go through the below links
Duplex Heater (Liquid- Liquid Heater) design calculation with online calculator
Hi friends Thanks for reading this article “Molasses cooler concepts”. I Hope you liked it. Give feed back, comments and please share it.

9 thoughts on “Molasses Cooler Design | Molasses Preservation | Properties of Molasses”
Reda kholef
(November 9, 2018 - 9:36 pm)Pleas need to calculated of prelimer capacity with 11000 beet ton /day .
Thank you
Kamal Singh Bisht
(March 27, 2021 - 8:52 am)The information provided are clear and concept right from basic, as Fire risk surveyor it helps me to deliver at least basic concept of molasses spontaneous combustion.
Thanks a Lot
siva alluri
(March 30, 2021 - 5:46 am)Welcome
Rajaram Patil
(November 13, 2018 - 2:52 pm)Very useful information, thanks.
siva alluri
(November 13, 2018 - 4:03 pm)Thank you Mr.Rajaram Patil
Carpio
(April 11, 2019 - 3:00 pm)Quote
Cooler for plate-type molasses to cool 20,053 kg / hr of molasses to 85 brix from 68 to 40 celsius
Quote FOB and CIF Puerto Cortes Honduras
Regards
siva alluri
(April 13, 2019 - 5:31 pm)Dear Sir,
It is only knowledge based website not a vendor site
Uday Pratap Singh
(July 6, 2019 - 4:10 am)Dear Sir,
Cooling of Syrup 50 Tonne per hour from 65 degree to 35 degree cooling cooling area required.Syrup brix 60.
Please help in calculation.
Dinesh Sharma
(June 28, 2021 - 11:14 am)Help me for calculating molasses cooler
10 M T per B heavy molasses