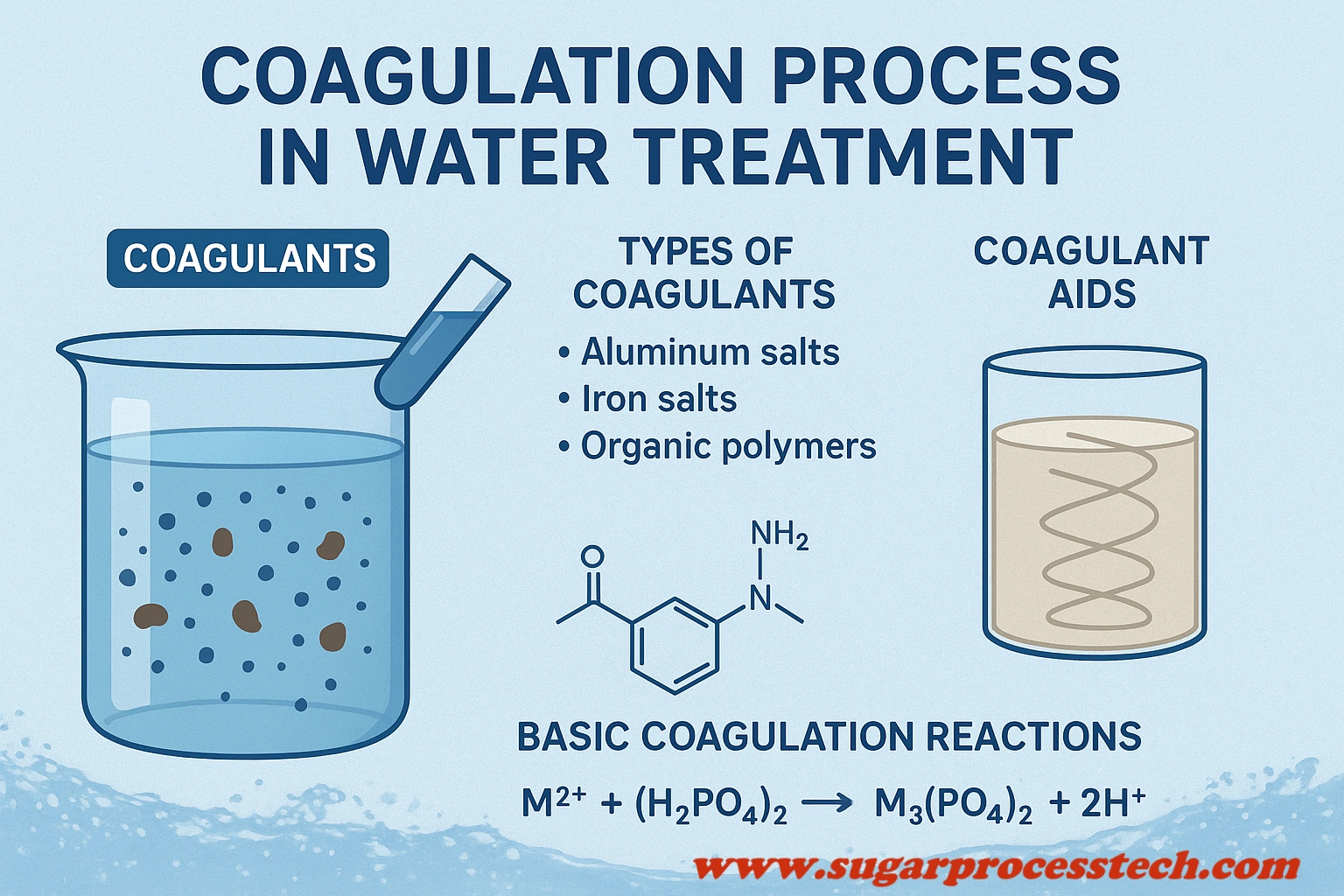
The coagulation process in water treatment plays an important role in the effective process of water treatment. It involves the use of coagulants and coagulant aids to remove impurities and particles from water. This article provides a complete guide, the coagulation process, types of coagulants, including their characteristics and applications, the significance of coagulant aids with examples and characteristics, basic coagulation reactions, coagulant dosage calculations, and more.
Understanding Coagulation Process:
Coagulation is the process of destabilizing repulsive charges on particles suspended in water, allowing them to clump together. These clumps are known as flocs, and they are formed when coagulants are introduced into the water.
Coagulants are chemicals that play an essential role and the initial step of the water and wastewater treatment process.
The Coagulants can be categorized into two main types, and they are primary coagulants & coagulant aids.
Primary Coagulants:
These chemicals neutralize the charges on particles and promote their aggregation. These coagulants include salts of iron or aluminum.
Examples of Coagulants, including the formulas and their Characteristics & Applications
| Coagulant | Chemical Formula | Characteristics and Applications |
|---|---|---|
| Aluminum Sulfate | Al2(SO4)3 · 14(H2O) | Characteristics: – Commonly known as Filter Alum. Forms aluminum hydroxide flocs that effectively trap impurities. Often used with cationic polymers to enhance coagulation.
Applications: – Effective in treating turbid water. Widely used in municipal and industrial water treatment processes where pH control is feasible. |
| Aluminum Chlorohydrate | Al2Cl(OH)5 | Characteristics: – Produces less sludge compared to some other coagulants – Can be corrosive when handled in concentrated form
Applications: – Suitable for water treatment applications where sludge generation needs to be minimized. Care should be taken when handling due to its corrosive nature. |
| Ferric Chloride | FeCl3 | Characteristics: – Effective coagulant over a wider pH range compared to alum.
Applications: – Used in the treatment of wastewater and industrial effluents – Particularly effective in situations where pH control is challenging. |
| Ferric Sulfate | Fe2(SO4)3 | Characteristics: – Often used in conjunction with lime softening processes.
Applications: – Effective in treating water with high alkalinity or hardness. Commonly employed in drinking water treatment for the removal of contaminants. |
| Ferrous Sulfate | Fe2(SO4)3 · 7H2O | Characteristics: – Less pH dependent compared to alum, making it suitable for a wider pH range of water
Applications: – Used in treating wastewater and in the removal of phosphates from water bodies. Offers flexibility in water treatment due to its lower pH dependence. |
| Sodium Aluminate | Na2Al2O4 | Characteristics: – Improves the coagulation efficiency of alum
Applications: – Enhances the performance of alum-based coagulation processes. Particularly useful when dealing with challenging water conditions where traditional alum treatment may be less effective. |
Coagulation Reactions in Water Treatment
These coagulants react with various components in the water to create insoluble particles or flocs. Here are the key coagulation reactions involving some common coagulants.
Aluminum Sulfate (Alum) Coagulation Reaction:
Al₂(SO₄)₃ + 6NaHCO₃ → 2Al(OH)₃ + 3Na₂SO₄ + 6CO₂
In this reaction, aluminum sulfate reacts with sodium bicarbonate in the water to form aluminum hydroxide, sodium sulfate, and carbon dioxide. Aluminum hydroxide particles serve as the nuclei for the formation of flocs, which capture impurities.
Ferric Sulfate Coagulation Reaction:
4Fe(OH)₂ + O₂ + 2H₂O → 4Fe(OH)₃
Ferric sulfate reacts with oxygen and water to produce ferric hydroxide. Ferric hydroxide particles aid in the aggregation of suspended solids, facilitating their removal during subsequent processes.
Ferrous Sulfate Coagulation Reaction:
FeSO₄ + Ca(OH)₂ → Fe(OH)₂ + CaSO₄
In this reaction, ferrous sulfate reacts with calcium hydroxide to form ferrous hydroxide and calcium sulfate. Ferrous hydroxide acts as a coagulant, promoting the formation of flocs.
Aluminum Sulfate (Alum) Coagulation Reaction with Calcium Hydroxide:
Al₂(SO₄)₃ + 3Ca(OH)₂ → 2Al(OH)₃ + CaSO₄
In this reaction, aluminum sulfate (Al₂(SO₄)₃), commonly known as alum, interacts with calcium hydroxide (Ca(OH)₂). The outcome of this chemical interaction is the formation of aluminum hydroxide (Al(OH)₃) and calcium sulfate (CaSO₄). Aluminum hydroxide serves as an effective coagulant by creating flocs that capture and agglomerate impurities present in the water. The resulting calcium sulfate is typically less soluble and may precipitate out of the water, further aiding in the removal of impurities.
Ferric Sulfate Coagulation Reaction with Calcium Bicarbonate:
Fe₂(SO₄)₃ + 3Ca(HCO₃)₂ → 2Fe(OH)₃ + 3CaSO₄ + 6CO₂
This reaction involves ferric sulfate (Fe₂(SO₄)₃) reacting with calcium bicarbonate (Ca(HCO₃)₂). The chemical outcome includes the formation of ferric hydroxide (Fe(OH)₃), calcium sulfate (CaSO₄), and the release of carbon dioxide (CO₂). Ferric hydroxide plays a vital role as a coagulant, facilitating the aggregation of suspended solids and impurities. Calcium sulfate is less soluble and can precipitate out, aiding in the removal of contaminants from the water. The release of carbon dioxide contributes to changes in water chemistry and may influence pH levels.
Coagulant Aids
Coagulant Aids are chemical substances that are used to increase or accelerate the Coagulation Process. It is also called a flocculant aid. Coagulant aids are enhancing the density and toughness of flocs, preventing them from breaking apart during successive water treatment processes. Polyelectrolytes are used as Coagulant aids.
Type of Coagulant aids with examples and characteristics.
| Polymer Type | Example | Characteristics |
|---|---|---|
| Cationic Polymers | PolyDADMAC (Polydiallyldimethylammonium chloride) (C8H16NCl)n | Positively charged ammonium groups in monomeric units. These are Large molecules, synthetic polyelectrolytes |
| Anionic Polymers | Polyacrylic Acid (CH2-CHCO2H)n. | Negatively charged carboxylate groups in monomeric units |
| Non-Ionic Polymers | Polyethylene Oxide (Polyethylene Glycol) H−(O−CH2−CH2)n−OH | Lacks charged groups in monomeric units |
pH and Coagulation
Achieving optimal coagulation efficiency requires considering the pH level of the water. Each coagulant has a specific pH range at which it operates most effectively. The ideal pH ensures maximum coagulant precipitation and minimal floc solubility. However, the best pH value may vary based on the specific water characteristics. For instance, distilled water coagulates optimally at a pH of 5.5, while natural waters typically fall within a pH range of 5.7 to 7.8. Iron coagulants can be used across a broader pH range of 5.5 to 11.0.
-
- Aluminum Sulfate (Alum) operates most effectively within a pH range of 5.5 to 7.5.
- Ferrous Sulfate performs optimally in a pH range of 8.0 to 11.0.
- Ferric Sulfate is also most effective within a pH range of 8.0 to 11.0. However, for specialized color removal purposes, a lower pH range of 5.0 to 6.0 is preferred.
Calculating the quantity of coagulant dosing
The process of coagulant dosing involves the following factors like the color of the water, alkalinity, and the concentration of suspended solids.
Calculate Alum Dosage based on colored Water
Use the following formula to calculate the initial dosage of alum required for colored water:
![]()
Where:
-
- Dc = Alum dosage (mg/L)
- Co = Color of the water in degrees (Use a colorimeter or spectrophotometer to measure the color of the water sample. The instrument will provide a numerical value representing the color intensity)
Calculation of Coagulant Dosing Based on Alkalinity with Example:
Alkalinity Value: Measure the alkalinity of the water sample in milligrams per liter (mg/L) as CaCO₃ using an alkalinity test kit or titration method.
Considered as follows
-
- Measured Alkalinity = 50 mg/L as CaCO₃
- Stoichiometric Alkalinity Factor (for alum) = 1.25
- Coagulant Dosage Factor = 3.0 mg/L
Calculate the Alkalinity Ratio (AR):
AR = Measured Alkalinity / Stoichiometric Alkalinity Factor
AR = 50 mg/L / 1.25 = 40 mg/L
Note: The stoichiometric alkalinity factor is specific to the coagulant you are using. For aluminum sulfate (alum), the stoichiometric alkalinity factor is typically 1.25.
Calculate the Coagulant Dosage (Dc):
Dc = AR x Coagulant Dosage Factor
Dc = 40 mg/L x 3.0 mg/L = 120 mg/L
Note: The Coagulant Dosage Factor varies depending on the specific water treatment plant and coagulant being used. It is determined through pilot testing or historical data. For this example, let’s assume the Coagulant Dosage Factor is 3.0 mg/L.
Convert Dosage to milligrams per cubic meter (mg/m³):
We know that 1 liter is equal to 0.001 cubic meters (1 L = 0.001 m³) and that the density of water is approximately 1000 kg/m³.
To convert from mg/L to mg/m³, we’ll multiply by the density of water:
Dc (mg/m³) = Dc (mg/L) * (1 L / 0.001 m³) * (1 kg / 1000 g)
Dc (mg/m³) = 0.12 mg/m³
Hence, based on the alkalinity of 50 mg/L as CaCO₃, a Coagulant Dosage Factor of 3.0 mg/L, and considering the density of water, you would add approximately 0.12 mg/m³ of coagulant to the water to achieve effective coagulation while considering the water’s alkalinity.
Calculation of Coagulant Dosage Based on Turbidity with Example
Turbidity Value: Measure the turbidity of the water sample using a turbidimeter. Let’s say you measure a turbidity of 20 nephelometric turbidity units (NTU).
Take one example:
-
- Measured Turbidity = 20 NTU (Nephelometric Turbidity Units)
- Stoichiometric Turbidity Factor (for alum) = 2.0
- Coagulant Dosage Factor = 1.5 mg/L
Determine the Turbidity Ratio (TR):
TR = Measured Turbidity / Stoichiometric Turbidity Factor
TR = 20 NTU / 2.0 = 10 NTU
Note: Calculate the turbidity ratio (TR) by dividing the measured turbidity by the stoichiometric turbidity factor for the coagulant being used. For example, let’s use aluminum sulfate (alum) with a stoichiometric turbidity factor of 2.0
Calculate the Coagulant Dosage (Dc) in milligrams per liter (mg/L):
Dc = TR x Coagulant Dosage Factor
Dc = 10 NTU x 1.5 mg/L = 15 mg/L
Note: The Coagulant Dosage Factor can vary depending on your specific water treatment plant and coagulant. For this example, assume a Coagulant Dosage Factor of 1.5 mg/L
Summary: The article explores the essential role of the coagulation process in water treatment. It covers the use of coagulants and coagulant aids to remove impurities from water. The primary coagulants, including their characteristics and applications, are systematically discussed. Additionally, key coagulation reactions involving common coagulants are explained. The article also provides a detailed guide on calculating coagulant dosages based on factors like water color, alkalinity, and turbidity.
Thank you for reading this article on water treatment coagulation. We hope you found it helpful. If you have any questions or need further information, please don’t hesitate to reach out. Your feedback is important to us. Leave a comment below.
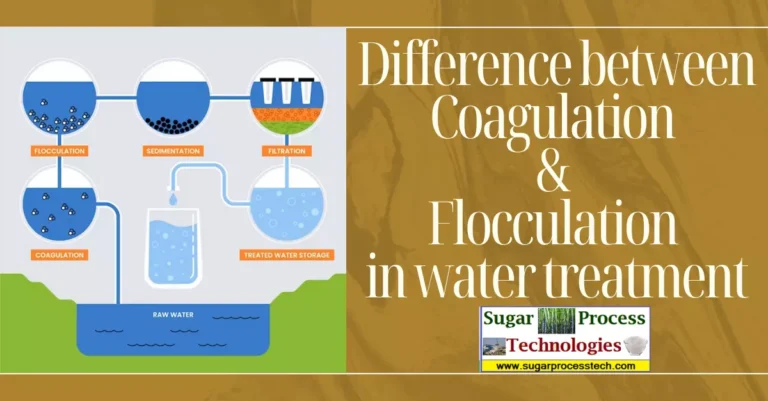
Difference between Coagulation and Flocculation in Water Treatment
Classification of Impurities Present in Water Related to Industrial Application
Chemical Oxygen Demand (COD): Meaning, Applications, and Control Strategies
Basic concepts of the Raw Water treatment system used in industrial applications
ETP | Sugar industry effluent treatment plant process philosophies
Humidity, Relative Humidity, Absolute Humidity, Specific Humidity & Dew Point
Activated Sludge Process for Wastewater Treatment | Anaerobic Digestion
Anaerobic Treatment Process for Industrial Waste Water | Anaerobic Digester
CSTR | Continuous Stirred Tank Reactor Design Criteria | Anaerobic Digester